Key figures
On this table, when a statistical test has been performed, the data showing a significant difference is displayed on a yellow background, otherwise on a grey background.
Data
ATC codes selected for analysis
| CODES (ATC-5) | LABELS |
| A01AA | CARIES PROPHYLACTIC AGENTS |
| A01AB | ANTIINFECTIVES AND ANTISEPTICS FOR LOCAL ORAL TREATMENT |
| A01AC | CORTICOSTEROIDS FOR LOCAL ORAL TREATMENT |
| A01AD | OTHER AGENTS FOR LOCAL ORAL TREATMENT |
| A02AB | ALUMINIUM COMPOUNDS |
| A02AC | CALCIUM COMPOUNDS |
| A02AD | COMBINATIONS AND COMPLEXES OF ALUMINIUM, CALCIUM AND MAGNESIUM COMPOUNDS |
| A02AF | ANTACIDS WITH ANTIFLATULENTS |
| A02AG | ANTACIDS WITH ANTISPASMODICS |
| A02AH | ANTACIDS WITH SODIUM BICARBONATE |
| A02AX | ANTACIDS, OTHER COMBINATIONS |
| A02BA | H2-RECEPTOR ANTAGONISTS |
| A02BB | PROSTAGLANDINS |
| A02BC | PROTON PUMP INHIBITORS |
| A02BD | COMBINATIONS FOR ERADICATION OF HELICOBACTER PYLORI |
| A02BX | OTHER DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) |
| A02DA | ANTIFLATULENTS |
| A03A | DRUGS FOR FUNCTIONAL BOWEL DISORDERS |
| A03AA | SYNTHETIC ANTICHOLINERGICS, ESTERS WITH TERTIARY AMINO GROUP |
| A03AB | SYNTHETIC ANTICHOLINERGICS, QUATERNARY AMMONIUM COMPOUNDS |
| A03AD | PAPAVERINE AND DERIVATIVES |
| A03AX | OTHER DRUGS FOR FUNCTIONAL BOWEL DISORDERS |
| A03BA | BELLADONNA ALKALOIDS, TERTIARY AMINES |
| A03BB | BELLADONNA ALKALOIDS, SEMISYNTHETIC, QUATERNARY AMMONIUM COMPOUNDS |
| A03CA | SYNTHETIC ANTICHOLINERGIC AGENTS IN COMBINATION WITH PSYCHOLEPTICS |
| A03CB | BELLADONNA AND DERIVATIVES IN COMBINATION WITH PSYCHOLEPTICS |
| A03DA | SYNTHETIC ANTICHOLINERGIC AGENTS IN COMBINATION WITH ANALGESICS |
| A03DB | BELLADONNA AND DERIVATIVES IN COMBINATION WITH ANALGESICS |
| A03FA | PROPULSIVES |
| A04AA | SEROTONIN (5HT3) ANTAGONISTS |
| A04AD | OTHER ANTIEMETICS |
| A05AA | BILE ACID PREPARATIONS |
| A05AX | OTHER DRUGS FOR BILE THERAPY |
| A05BA | LIVER THERAPY |
| A06 | LAXATIVES |
| A06AA | SOFTENERS, EMOLLIENTS |
| A06AB | CONTACT LAXATIVES |
| A06AC | BULK PRODUCERS |
| A06AD | OSMOTICALLY ACTING LAXATIVES |
| A06AG | ENEMAS |
| A06AH | PERIPHERAL OPIOID RECEPTOR ANTAGONISTS |
| A06AX | OTHER LAXATIVES |
| A07AA | ANTIBIOTICS |
| A07AB | SULFONAMIDES |
| A07AC | IMIDAZOLE DERIVATIVES |
| A07AX | OTHER INTESTINAL ANTIINFECTIVES |
| A07BA | CHARCOAL PREPARATIONS |
| A07BB | BISMUTH PREPARATIONS |
| A07BC | OTHER INTESTINAL ADSORBENTS |
| A07DA | ANTIPROPULSIVES |
| A07EA | CORTICOSTEROIDS ACTING LOCALLY |
| A07EC | AMINOSALICYLIC ACID AND SIMILAR AGENTS |
| A07FA | ANTIDIARRHEAL MICROORGANISMS |
| A07XA | OTHER ANTIDIARRHEALS |
| A08AA | CENTRALLY ACTING ANTIOBESITY PRODUCTS |
| A08AB | PERIPHERALLY ACTING ANTIOBESITY PRODUCTS |
| A08AX | OTHER ANTIOBESITY DRUGS |
| A09AA | ENZYME PREPARATIONS |
| A09AC | ENZYME AND ACID PREPARATIONS, COMBINATIONS |
| A10AB | INSULINS AND ANALOGUES FOR INJECTION, FAST-ACTING |
| A10AC | INSULINS AND ANALOGUES FOR INJECTION, INTERMEDIATE-ACTING |
| A10AD | INSULINS AND ANALOGUES FOR INJECTION, INTERMEDIATE-ACTING COMBINED WITH FAST-ACTING |
| A10AE | INSULINS AND ANALOGUES FOR INJECTION, LONG-ACTING |
| A10BA | BIGUANIDES |
| A10BB | SULFONAMIDES, UREA DERIVATIVES |
| A10BD | COMBINATIONS OF ORAL BLOOD GLUCOSE LOWERING DRUGS |
| A10BF | ALPHA GLUCOSIDASE INHIBITORS |
| A10BG | THIAZOLIDINEDIONES |
| A10BH | DIPEPTIDYL PEPTIDASE 4 (DPP-4) INHIBITORS |
| A10BJ | GLUCAGON-LIKE PEPTIDE-1 (GLP-1) ANALOGUES |
| A10BK | SODIUM-GLUCOSE CO-TRANSPORTER 2 (SGLT2) INHIBITORS |
| A10BX | OTHER BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS |
| A11AA | MULTIVITAMINS WITH MINERALS |
| A11BA | MULTIVITAMINS, PLAIN |
| A11CA | VITAMIN A, PLAIN |
| A11CB | VITAMIN A AND D IN COMBINATION |
| A11CC | VITAMIN D AND ANALOGUES |
| A11DA | VITAMIN B1, PLAIN |
| A11DB | VITAMIN B1 IN COMBINATION WITH VITAMIN B6 AND/OR VITAMIN B12 |
| A11EA | VITAMIN B-COMPLEX, PLAIN |
| A11EB | VITAMIN B-COMPLEX WITH VITAMIN C |
| A11EX | VITAMIN B-COMPLEX, OTHER COMBINATIONS |
| A11GA | ASCORBIC ACID (VITAMIN C), PLAIN |
| A11HA | OTHER PLAIN VITAMIN PREPARATIONS |
| A11JA | COMBINATIONS OF VITAMINS |
| A11JC | VITAMINS, OTHER COMBINATIONS |
| A12AA | CALCIUM |
| A12AX | CALCIUM, COMBINATIONS WITH OTHER DRUGS |
| A12BA | POTASSIUM |
| A12CA | SODIUM |
| A12CB | ZINC |
| A12CC | MAGNESIUM |
| A12CD | FLUORIDE |
| A12CE | SELENIUM |
| A12CX | OTHER MINERAL PRODUCTS |
| A13A | TONICS |
| A14AA | ANDROSTAN DERIVATIVES |
| A14AB | ESTREN DERIVATIVES |
| A16AA | AMINO ACIDS AND DERIVATIVES |
| A16AB | ENZYMES |
| A16AX | VARIOUS ALIMENTARY TRACT AND METABOLISM PRODUCTS |
| B01AA | VITAMIN K ANTAGONISTS |
| B01AB | HEPARIN GROUP |
| B01AC | PLATELET AGGREGATION INHIBITORS EXCL. HEPARIN |
| B01AD | ENZYMES |
| B01AE | DIRECT THROMBIN INHIBITORS |
| B01AF | DIRECT FACTOR XA INHIBITORS |
| B01AX | OTHER ANTITHROMBOTIC AGENTS |
| B02AA | AMINO ACIDS |
| B02AB | PROTEINASE INHIBITORS |
| B02BA | VITAMIN K |
| B02BB | FIBRINOGEN |
| B02BC | LOCAL HEMOSTATICS |
| B02BD | BLOOD COAGULATION FACTORS |
| B02BX | OTHER SYSTEMIC HEMOSTATICS |
| B03AA | IRON BIVALENT, ORAL PREPARATIONS |
| B03AB | IRON TRIVALENT, ORAL PREPARATIONS |
| B03AC | IRON TRIVALENT, PARENTERAL PREPARATIONS |
| B03AD | IRON IN COMBINATION WITH FOLIC ACID |
| B03AE | IRON IN OTHER COMBINATIONS |
| B03BA | VITAMIN B12 (CYANOCOBALAMIN AND ANALOGUES) |
| B03BB | FOLIC ACID AND DERIVATIVES |
| B03XA | OTHER ANTIANEMIC PREPARATIONS |
| B05 | BLOOD SUBSTITUTES AND PERFUSION SOLUTIONS |
| B05AA | BLOOD SUBSTITUTES AND PLASMA PROTEIN FRACTIONS |
| B05BA | SOLUTIONS FOR PARENTERAL NUTRITION |
| B05BB | SOLUTIONS AFFECTING THE ELECTROLYTE BALANCE |
| B05BC | SOLUTIONS PRODUCING OSMOTIC DIURESIS |
| B05CA | ANTIINFECTIVES |
| B05CB | SALT SOLUTIONS |
| B05CX | OTHER IRRIGATING SOLUTIONS |
| B05DB | HYPERTONIC SOLUTIONS |
| B05X | I.V. SOLUTION ADDITIVES |
| B05XA | ELECTROLYTE SOLUTIONS |
| B05XB | AMINO ACIDS |
| B05XC | VITAMINS |
| B05ZB | HEMOFILTRATES |
| B06AA | ENZYMES |
| B06AB | OTHER HEM PRODUCTS |
| B06AC | DRUGS USED IN HEREDITARY ANGIOEDEMA |
| C01AA | DIGITALIS GLYCOSIDES |
| C01BA | ANTIARRHYTHMICS, CLASS IA |
| C01BB | ANTIARRHYTHMICS, CLASS IB |
| C01BC | ANTIARRHYTHMICS, CLASS IC |
| C01BD | ANTIARRHYTHMICS, CLASS III |
| C01BG | OTHER CLASS I ANTIARRHYTHMICS |
| C01CA | ADRENERGIC AND DOPAMINERGIC AGENTS |
| C01CE | PHOSPHODIESTERASE INHIBITORS |
| C01CX | OTHER CARDIAC STIMULANTS |
| C01DA | ORGANIC NITRATES |
| C01DX | OTHER VASODILATORS USED IN CARDIAC DISEASES |
| C01EA | PROSTAGLANDINS |
| C01EB | OTHER CARDIAC PREPARATIONS |
| C02AA | RAUWOLFIA ALKALOIDS |
| C02AB | METHYLDOPA |
| C02AC | IMIDAZOLINE RECEPTOR AGONISTS |
| C02CA | ALPHA-ADRENORECEPTOR ANTAGONISTS |
| C02CC | GUANIDINE DERIVATIVES |
| C02DA | THIAZIDE DERIVATIVES |
| C02DB | HYDRAZINOPHTHALAZINE DERIVATIVES |
| C02DC | PYRIMIDINE DERIVATIVES |
| C02DD | NITROFERRICYANIDE DERIVATIVES |
| C02DG | GUANIDINE DERIVATIVES |
| C02KD | SEROTONIN ANTAGONISTS |
| C02KX | OTHER ANTIHYPERTENSIVES |
| C03AA | THIAZIDES, PLAIN |
| C03AB | THIAZIDES AND POTASSIUM IN COMBINATION |
| C03BA | SULFONAMIDES, PLAIN |
| C03CA | SULFONAMIDES, PLAIN |
| C03DA | ALDOSTERONE ANTAGONISTS |
| C03DB | OTHER POTASSIUM-SPARING AGENTS |
| C03EA | LOW-CEILING DIURETICS AND POTASSIUM-SPARING AGENTS |
| C03EB | HIGH-CEILING DIURETICS AND POTASSIUM-SPARING AGENTS |
| C03XA | VASOPRESSIN ANTAGONISTS |
| C04AA | 2-AMINO-1-PHENYLETHANOL DERIVATIVES |
| C04AB | IMIDAZOLINE DERIVATIVES |
| C04AC | NICOTINIC ACID AND DERIVATIVES |
| C04AD | PURINE DERIVATIVES |
| C04AE | ERGOT ALKALOIDS |
| C04AX | OTHER PERIPHERAL VASODILATORS |
| C05AA | CORTICOSTEROIDS |
| C05AD | LOCAL ANESTHETICS |
| C05AE | MUSCLE RELAXANTS |
| C05AX | OTHER AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE |
| C05BA | HEPARINS OR HEPARINOIDS FOR TOPICAL USE |
| C05BB | SCLEROSING AGENTS FOR LOCAL INJECTION |
| C05BX | OTHER SCLEROSING AGENTS |
| C05CA | BIOFLAVONOIDS |
| C05CX | OTHER CAPILLARY STABILIZING AGENTS |
| C07AA | BETA BLOCKING AGENTS, NON-SELECTIVE |
| C07AB | BETA BLOCKING AGENTS, SELECTIVE |
| C07AG | ALPHA AND BETA BLOCKING AGENTS |
| C07BA | BETA BLOCKING AGENTS, NON-SELECTIVE, AND THIAZIDES |
| C07BB | BETA BLOCKING AGENTS, SELECTIVE, AND THIAZIDES |
| C07CA | BETA BLOCKING AGENTS, NON-SELECTIVE, AND OTHER DIURETICS |
| C07CB | BETA BLOCKING AGENTS, SELECTIVE, AND OTHER DIURETICS |
| C07DB | BETA BLOCKING AGENTS, SELECTIVE, THIAZIDES AND OTHER DIURETICS |
| C07FB | BETA BLOCKING AGENTS, SELECTIVE, AND OTHER ANTIHYPERTENSIVES |
| C08CA | DIHYDROPYRIDINE DERIVATIVES |
| C08DA | PHENYLALKYLAMINE DERIVATIVES |
| C08DB | BENZOTHIAZEPINE DERIVATIVES |
| C08EA | PHENYLALKYLAMINE DERIVATIVES |
| C08EX | OTHER NON-SELECTIVE CALCIUM CHANNEL BLOCKERS |
| C09AA | ACE INHIBITORS, PLAIN |
| C09BA | ACE INHIBITORS AND DIURETICS |
| C09BB | ACE INHIBITORS AND CALCIUM CHANNEL BLOCKERS |
| C09BX | ACE INHIBITORS, OTHER COMBINATIONS |
| C09CA | ANGIOTENSIN II ANTAGONISTS, PLAIN |
| C09DA | ANGIOTENSIN II ANTAGONISTS AND DIURETICS |
| C09DB | ANGIOTENSIN II ANTAGONISTS AND CALCIUM CHANNEL BLOCKERS |
| C09DX | ANGIOTENSIN II ANTAGONISTS, OTHER COMBINATIONS |
| C09XA | RENIN-INHIBITORS |
| C10AA | HMG COA REDUCTASE INHIBITORS |
| C10AB | FIBRATES |
| C10AC | BILE ACID SEQUESTRANTS |
| C10AD | NICOTINIC ACID AND DERIVATIVES |
| C10AX | OTHER LIPID MODIFYING AGENTS |
| C10BA | COMBINATIONS OF VARIOUS LIPID MODIFYING AGENTS |
| C10BX | LIPID MODIFYING AGENTS IN COMBINATION WITH OTHER DRUGS |
| D | DERMATOLOGICALS |
| D01AA | ANTIBIOTICS |
| D01AC | IMIDAZOLE AND TRIAZOLE DERIVATIVES |
| D01AE | OTHER ANTIFUNGALS FOR TOPICAL USE |
| D01BA | ANTIFUNGALS FOR SYSTEMIC USE |
| D02AB | ZINC PRODUCTS |
| D02AD | LIQUID PLASTERS |
| D02AE | CARBAMIDE PRODUCTS |
| D02AF | SALICYLIC ACID PREPARATIONS |
| D02AX | OTHER EMOLLIENTS AND PROTECTIVES |
| D03AA | COD-LIVER OIL OINTMENTS |
| D03AX | OTHER CICATRIZANTS |
| D03BA | PROTEOLYTIC ENZYMES |
| D04AA | ANTIHISTAMINES FOR TOPICAL USE |
| D04AB | ANESTHETICS FOR TOPICAL USE |
| D04AX | OTHER ANTIPRURITICS |
| D05AA | TARS |
| D05AC | ANTRACEN DERIVATIVES |
| D05AX | OTHER ANTIPSORIATICS FOR TOPICAL USE |
| D05BA | PSORALENS FOR SYSTEMIC USE |
| D05BB | RETINOIDS FOR TREATMENT OF PSORIASIS |
| D06AA | TETRACYCLINE AND DERIVATIVES |
| D06AX | OTHER ANTIBIOTICS FOR TOPICAL USE |
| D06BA | SULFONAMIDES |
| D06BB | ANTIVIRALS |
| D06BX | OTHER CHEMOTHERAPEUTICS |
| D07AA | CORTICOSTEROIDS, WEAK (GROUP I) |
| D07AB | CORTICOSTEROIDS, MODERATELY POTENT (GROUP II) |
| D07AC | CORTICOSTEROIDS, POTENT (GROUP III) |
| D07AD | CORTICOSTEROIDS, VERY POTENT (GROUP IV) |
| D07BB | CORTICOSTEROIDS, MODERATELY POTENT, COMBINATIONS WITH ANTISEPTICS |
| D07BC | CORTICOSTEROIDS, POTENT, COMBINATIONS WITH ANTISEPTICS |
| D07CA | CORTICOSTEROIDS, WEAK, COMBINATIONS WITH ANTIBIOTICS |
| D07CB | CORTICOSTEROIDS, MODERATELY POTENT, COMBINATIONS WITH ANTIBIOTICS |
| D07CC | CORTICOSTEROIDS, POTENT, COMBINATIONS WITH ANTIBIOTICS |
| D07XA | CORTICOSTEROIDS, WEAK, OTHER COMBINATIONS |
| D07XB | CORTICOSTEROIDS, MODERATELY POTENT, OTHER COMBINATIONS |
| D07XC | CORTICOSTEROIDS, POTENT, OTHER COMBINATIONS |
| D08AB | ALUMINIUM AGENTS |
| D08AC | BIGUANIDES AND AMIDINES |
| D08AD | BORIC ACID PRODUCTS |
| D08AE | PHENOL AND DERIVATIVES |
| D08AF | NITROFURAN DERIVATIVES |
| D08AG | IODINE PRODUCTS |
| D08AH | QUINOLINE DERIVATIVES |
| D08AJ | QUATERNARY AMMONIUM COMPOUNDS |
| D08AK | MERCURIAL PRODUCTS |
| D08AL | SILVER COMPOUNDS |
| D08AX | OTHER ANTISEPTICS AND DISINFECTANTS |
| D09AA | MEDICATED DRESSINGS WITH ANTIINFECTIVES |
| D09AB | ZINC BANDAGES |
| D09AX | SOFT PARAFFIN DRESSINGS |
| D10AD | RETINOIDS FOR TOPICAL USE IN ACNE |
| D10AE | PEROXIDES |
| D10AF | ANTIINFECTIVES FOR TREATMENT OF ACNE |
| D10AX | OTHER ANTI-ACNE PREPARATIONS FOR TOPICAL USE |
| D10BA | RETINOIDS FOR TREATMENT OF ACNE |
| D11AC | MEDICATED SHAMPOOS |
| D11AF | WART AND ANTI-CORN PREPARATIONS |
| D11AH | AGENTS FOR ATOPIC DERMATITIS, EXCLUDING CORTICOSTEROIDS |
| D11AX | OTHER DERMATOLOGICALS |
| G01AA | ANTIBIOTICS |
| G01AC | QUINOLINE DERIVATIVES |
| G01AD | ORGANIC ACIDS |
| G01AE | SULFONAMIDES |
| G01AF | IMIDAZOLE DERIVATIVES |
| G01AG | TRIAZOLE DERIVATIVES |
| G01AX | OTHER ANTIINFECTIVES AND ANTISEPTICS |
| G02AB | ERGOT ALKALOIDS |
| G02AD | PROSTAGLANDINS |
| G02BA | INTRAUTERINE CONTRACEPTIVES |
| G02BB | INTRAVAGINAL CONTRACEPTIVES |
| G02CA | SYMPATHOMIMETICS, LABOUR REPRESSANTS |
| G02CB | PROLACTINE INHIBITORS |
| G02CX | OTHER GYNECOLOGICALS |
| G03AA | PROGESTOGENS AND ESTROGENS, FIXED COMBINATIONS |
| G03AB | PROGESTOGENS AND ESTROGENS, SEQUENTIAL PREPARATIONS |
| G03AC | PROGESTOGENS |
| G03AD | EMERGENCY CONTRACEPTIVES |
| G03BA | 3-OXOANDROSTEN (4) DERIVATIVES |
| G03BB | 5-ANDROSTANON (3) DERIVATIVES |
| G03CA | NATURAL AND SEMISYNTHETIC ESTROGENS, PLAIN |
| G03CB | SYNTHETIC ESTROGENS, PLAIN |
| G03CC | ESTROGENS, COMBINATIONS WITH OTHER DRUGS |
| G03CX | OTHER ESTROGENS |
| G03DA | PREGNEN (4) DERIVATIVES |
| G03DB | PREGNADIEN DERIVATIVES |
| G03DC | ESTREN DERIVATIVES |
| G03EA | ANDROGENS AND ESTROGENS |
| G03FA | PROGESTOGENS AND ESTROGENS, FIXED COMBINATIONS |
| G03FB | PROGESTOGENS AND ESTROGENS, SEQUENTIAL PREPARATIONS |
| G03GA | GONADOTROPINS |
| G03GB | OVULATION STIMULANTS, SYNTHETIC |
| G03HA | ANTIANDROGENS, PLAIN |
| G03HB | ANTIANDROGENS AND ESTROGENS |
| G03XA | ANTIGONADOTROPINS AND SIMILAR AGENTS |
| G03XB | ANTIPROGESTOGENS |
| G03XC | SELECTIVE ESTROGEN RECEPTOR MODULATORS |
| G03XX | OTHER SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM |
| G04BA | ACIDIFIERS |
| G04BC | URINARY CONCREMENT SOLVENTS |
| G04BD | URINARY ANTISPASMODICS |
| G04BE | DRUGS USED IN ERECTILE DYSFUNCTION |
| G04BX | OTHER UROLOGICALS |
| G04CA | ALPHA-ADRENORECEPTOR ANTAGONISTS |
| G04CB | TESTOSTERONE-5-ALPHA REDUCTASE INHIBITORS |
| G04CX | OTHER DRUGS USED IN BENIGN PROSTATIC HYPERTROPHY |
| H01AA | ACTH |
| H01AC | SOMATROPIN AND SOMATROPIN AGONISTS |
| H01AX | OTHER ANTERIOR PITUITARY LOBE HORMONES AND ANALOGUES |
| H01BA | VASOPRESSIN AND ANALOGUES |
| H01BB | OXYTOCIN AND ANALOGUES |
| H01CA | GONADOTROPIN-RELEASING HORMONES |
| H01CB | ANTIGROWTH HORMONES |
| H01CC | ANTI-GONADOTROPIN-RELEASING HORMONES |
| H02AA | MINERALOCORTICOIDS |
| H02AB | GLUCOCORTICOIDS |
| H02BX | CORTICOSTEROIDS FOR SYSTEMIC USE, COMBINATIONS |
| H03AA | THYROID HORMONES |
| H03BA | THIOURACILS |
| H03BB | SULFUR-CONTAINING IMIDAZOLE DERIVATIVES |
| H04AA | GLYCOGENOLYTIC HORMONES |
| H05AA | PARATHYROID HORMONES AND ANALOGUES |
| H05BA | CALCITONIN PREPARATIONS |
| H05BX | OTHER ANTI-PARATHYROID AGENTS |
| J01 | ANTIBACTERIALS FOR SYSTEMIC USE |
| J01AA | TETRACYCLINES |
| J01BA | AMPHENICOLS |
| J01CA | PENICILLINS WITH EXTENDED SPECTRUM |
| J01CE | BETA-LACTAMASE SENSITIVE PENICILLINS |
| J01CF | BETA-LACTAMASE RESISTANT PENICILLINS |
| J01CR | COMBINATIONS OF PENICILLINS, INCL. BETA-LACTAMASE INHIBITORS |
| J01DB | FIRST-GENERATION CEPHALOSPORINS |
| J01DC | SECOND-GENERATION CEPHALOSPORINS |
| J01DD | THIRD-GENERATION CEPHALOSPORINS |
| J01DE | FOURTH-GENERATION CEPHALOSPORINS |
| J01DF | MONOBACTAMS |
| J01DH | CARBAPENEMS |
| J01DI | OTHER CEPHALOSPORINS |
| J01EA | TRIMETHOPRIM AND DERIVATIVES |
| J01EB | SHORT-ACTING SULFONAMIDES |
| J01EC | INTERMEDIATE-ACTING SULFONAMIDES |
| J01ED | LONG-ACTING SULFONAMIDES |
| J01EE | COMBINATIONS OF SULFONAMIDES AND TRIMETHOPRIM, INCL. DERIVATIVES |
| J01FA | MACROLIDES |
| J01FF | LINCOSAMIDES |
| J01FG | STREPTOGRAMINS |
| J01GA | STREPTOMYCINS |
| J01GB | OTHER AMINOGLYCOSIDES |
| J01MA | FLUOROQUINOLONES |
| J01MB | OTHER QUINOLONES |
| J01XA | GLYCOPEPTIDE ANTIBACTERIALS |
| J01XB | POLYMYXINS |
| J01XC | STEROID ANTIBACTERIALS |
| J01XD | IMIDAZOLE DERIVATIVES |
| J01XE | NITROFURAN DERIVATIVES |
| J01XX | OTHER ANTIBACTERIALS |
| J02AA | ANTIBIOTICS |
| J02AB | IMIDAZOLE DERIVATIVES |
| J02AC | TRIAZOLE DERIVATIVES |
| J02AX | OTHER ANTIMYCOTICS FOR SYSTEMIC USE |
| J04AB | ANTIBIOTICS |
| J04AC | HYDRAZIDES |
| J04AK | OTHER DRUGS FOR TREATMENT OF TUBERCULOSIS |
| J04BA | DRUGS FOR TREATMENT OF LEPRA |
| J05AB | NUCLEOSIDES AND NUCLEOTIDES EXCL. REVERSE TRANSCRIPTASE INHIBITORS |
| J05AD | PHOSPHONIC ACID DERIVATIVES |
| J05AE | PROTEASE INHIBITORS |
| J05AF | NUCLEOSIDE AND NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS |
| J05AG | NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS |
| J05AH | NEURAMINIDASE INHIBITORS |
| J05AJ | INTEGRASE INHIBITORS |
| J05AP | ANTIVIRALS FOR TREATMENT OF HCV INFECTIONS |
| J05AR | ANTIVIRALS FOR TREATMENT OF HIV INFECTIONS, COMBINATIONS |
| J05AX | OTHER ANTIVIRALS |
| J06AA | IMMUNE SERA |
| J06BA | IMMUNOGLOBULINS, NORMAL HUMAN |
| J06BB | SPECIFIC IMMUNOGLOBULINS |
| J06BD | ANTIVIRAL MONOCLONAL ANTIBODIES |
| J07 | VACCINES |
| J07AE | CHOLERA VACCINES |
| J07AG | HEMOPHILUS INFLUENZAE B VACCINES |
| J07AH | MENINGOCOCCAL VACCINES |
| J07AJ | PERTUSSIS VACCINES |
| J07AL | PNEUMOCOCCAL VACCINES |
| J07AM | TETANUS VACCINES |
| J07AP | TYPHOID VACCINES |
| J07AX | OTHER BACTERIAL VACCINES |
| J07BA | ENCEPHALITIS VACCINES |
| J07BB | INFLUENZA VACCINES |
| J07BC | HEPATITIS VACCINES |
| J07BD | MEASLES VACCINES |
| J07BE | MUMPS VACCINES |
| J07BF | POLIOMYELITIS VACCINES |
| J07BG | RABIES VACCINES |
| J07BH | ROTA VIRUS DIARRHEA VACCINES |
| J07BJ | RUBELLA VACCINES |
| J07BK | VARICELLA ZOSTER VACCINES |
| J07BL | YELLOW FEVER VACCINES |
| J07BM | PAPILLOMAVIRUS VACCINES |
| J07BN | Covid-19 vaccines |
| J07BX | OTHER VIRAL VACCINES |
| J07CA | BACTERIAL AND VIRAL VACCINES, COMBINED |
| L01AA | NITROGEN MUSTARD ANALOGUES |
| L01AB | ALKYL SULFONATES |
| L01AC | ETHYLENE IMINES |
| L01AD | NITROSOUREAS |
| L01AX | OTHER ALKYLATING AGENTS |
| L01BA | FOLIC ACID ANALOGUES |
| L01BB | PURINE ANALOGUES |
| L01BC | PYRIMIDINE ANALOGUES |
| L01CA | VINCA ALKALOIDS AND ANALOGUES |
| L01CB | PODOPHYLLOTOXIN DERIVATIVES |
| L01CD | TAXANES |
| L01CE | TOPOISOMERASE 1 (TOP1) INHIBITORS |
| L01CX | OTHER PLANT ALKALOIDS AND NATURAL PRODUCTS |
| L01DA | ACTINOMYCINES |
| L01DB | ANTHRACYCLINES AND RELATED SUBSTANCES |
| L01DC | OTHER CYTOTOXIC ANTIBIOTICS |
| L01EA | BCR-ABL TYROSINE KINASE INHIBITORS |
| L01EB | EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR) TYROSINE KINASE INHIBITORS |
| L01EC | B-RAF SERINE-THREONINE KINASE (BRAF) INHIBITORS |
| L01ED | ANAPLASTIC LYMPHOMA KINASE (ALK) INHIBITORS |
| L01EE | MITOGEN-ACTIVATED PROTEIN KINASE (MEK) INHIBITORS |
| L01EF | CYCLIN-DEPENDENT KINASE (CDK) INHIBITORS |
| L01EG | MAMMALIAN TARGET OF RAPAMYCIN (MTOR) KINASE INHIBITORS |
| L01EH | HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER2) TYROSINE KINASE INHIBITORS |
| L01EJ | JANUS-ASSOCIATED KINASE (JAK) INHIBITORS |
| L01EK | VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR (VEGFR) TYROSINE KINASE INHIBITORS |
| L01EL | BRUTON'S TYROSINE KINASE (BTK) INHIBITORS |
| L01EM | PHOSPHATIDYLINOSITOL-3-KINASE (PI3K) INHIBITORS |
| L01EN | FIBROBLAST GROWTH FACTOR RECEPTOR (FGFR) TYROSINE KINASE INHIBITORS |
| L01EX | OTHER PROTEIN KINASE INHIBITORS |
| L01FA | CD20 (CLUSTERS OF DIFFERENTIATION 20) INHIBITORS |
| L01FB | CD22 (CLUSTERS OF DIFFERENTIATION 22) INHIBITORS |
| L01FC | CD38 (CLUSTERS OF DIFFERENTIATION 38) INHIBITORS |
| L01FD | HER2 (HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2) INHIBITORS |
| L01FE | EGFR (EPIDERMAL GROWTH FACTOR RECEPTOR) INHIBITORS |
| L01FF | PD-1/PDL-1 (PROGRAMMED CELL DEATH PROTEIN 1/DEATH LIGAND 1) INHIBITORS |
| L01FG | VEGF/VEGFR (VASCULAR ENDOTHELIAL GROWTH FACTOR) INHIBITORS |
| L01FX | OTHER MONOCLONAL ANTIBODIES AND ANTIBODY DRUG CONJUGATES |
| L01XA | PLATINUM COMPOUNDS |
| L01XB | METHYLHYDRAZINES |
| L01XD | SENSITIZERS USED IN PHOTODYNAMIC/RADIATION THERAPY |
| L01XF | RETINOIDS FOR CANCER TREATMENT |
| L01XG | PROTEASOME INHIBITORS |
| L01XH | HISTONE DEACETYLASE (HDAC) INHIBITORS |
| L01XJ | HEDGEHOG PATHWAY INHIBITORS |
| L01XK | POLY (ADP-RIBOSE) POLYMERASE (PARP) INHIBITORS |
| L01XL | Antineoplastic cell and gene therapy |
| L01XX | OTHER ANTINEOPLASTIC AGENTS |
| L01XY | COMBINATIONS OF ANTINEOPLASTIC AGENTS |
| L02AA | ESTROGENS |
| L02AB | PROGESTOGENS |
| L02AE | GONADOTROPIN RELEASING HORMONE ANALOGUES |
| L02BA | ANTI-ESTROGENS |
| L02BB | ANTI-ANDROGENS |
| L02BG | ENZYME INHIBITORS |
| L02BX | OTHER HORMONE ANTAGONISTS AND RELATED AGENTS |
| L03AA | COLONY STIMULATING FACTORS |
| L03AB | INTERFERONS |
| L03AC | INTERLEUKINS |
| L03AX | OTHER IMMUNOSTIMULANTS |
| L04AA | SELECTIVE IMMUNOSUPPRESSANTS |
| L04AB | TUMOR NECROSIS FACTOR ALPHA (TNF-?) INHIBITORS |
| L04AC | INTERLEUKIN INHIBITORS |
| L04AD | CALCINEURIN INHIBITORS |
| L04AX | OTHER IMMUNOSUPPRESSANTS |
| M01AA | BUTYLPYRAZOLIDINES |
| M01AB | ACETIC ACID DERIVATIVES AND RELATED SUBSTANCES |
| M01AC | OXICAMS |
| M01AE | PROPIONIC ACID DERIVATIVES |
| M01AG | FENAMATES |
| M01AH | COXIBS |
| M01AX | OTHER ANTIINFLAMMATORY AND ANTIRHEUMATIC AGENTS, NON-STEROIDS |
| M01CB | GOLD PREPARATIONS |
| M01CC | PENICILLAMINE AND SIMILAR AGENTS |
| M02AA | ANTIINFLAMMATORY PREPARATIONS, NON-STEROIDS FOR TOPICAL USE |
| M02AB | CAPSAICIN AND SIMILAR AGENTS |
| M02AC | PREPARATIONS WITH SALICYLIC ACID DERIVATIVES |
| M02AX | OTHER TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN |
| M03AB | CHOLINE DERIVATIVES |
| M03AC | OTHER QUATERNARY AMMONIUM COMPOUNDS |
| M03AX | OTHER MUSCLE RELAXANTS, PERIPHERALLY ACTING AGENTS |
| M03BA | CARBAMIC ACID ESTERS |
| M03BB | OXAZOL, THIAZINE, AND TRIAZINE DERIVATIVES |
| M03BC | ETHERS, CHEMICALLY CLOSE TO ANTIHISTAMINES |
| M03BX | OTHER CENTRALLY ACTING AGENTS |
| M03CA | DANTROLENE AND DERIVATIVES |
| M04AA | PREPARATIONS INHIBITING URIC ACID PRODUCTION |
| M04AB | PREPARATIONS INCREASING URIC ACID EXCRETION |
| M04AC | PREPARATIONS WITH NO EFFECT ON URIC ACID METABOLISM |
| M05BA | BISPHOSPHONATES |
| M05BB | BISPHOSPHONATES, COMBINATIONS |
| M05BC | BONE MORPHOGENETIC PROTEINS |
| M05BX | OTHER DRUGS AFFECTING BONE STRUCTURE AND MINERALIZATION |
| M09AB | ENZYMES |
| M09AX | OTHER DRUGS FOR DISORDERS OF THE MUSCULO-SKELETAL SYSTEM |
| N01AB | HALOGENATED HYDROCARBONS |
| N01AF | BARBITURATES, PLAIN |
| N01AH | OPIOID ANESTHETICS |
| N01AX | OTHER GENERAL ANESTHETICS |
| N01BA | ESTERS OF AMINOBENZOIC ACID |
| N01BB | AMIDES |
| N01BX | OTHER LOCAL ANESTHETICS |
| N02AA | NATURAL OPIUM ALKALOIDS |
| N02AB | PHENYLPIPERIDINE DERIVATIVES |
| N02AC | DIPHENYLPROPYLAMINE DERIVATIVES |
| N02AD | BENZOMORPHAN DERIVATIVES |
| N02AE | ORIPAVINE DERIVATIVES |
| N02AJ | OPIOIDS IN COMBINATION WITH NON-OPIOID ANALGESICS |
| N02AX | OTHER OPIOIDS |
| N02BA | SALICYLIC ACID AND DERIVATIVES |
| N02BB | PYRAZOLONES |
| N02BE | ANILIDES |
| N02BG | OTHER ANALGESICS AND ANTIPYRETICS |
| N02CA | ERGOT ALKALOIDS |
| N02CC | SELECTIVE SEROTONIN (5HT1) AGONISTS |
| N02CD | CALCITONIN GENE-RELATED PEPTIDE (CGRP) ANTAGONISTS |
| N02CX | OTHER ANTIMIGRAINE PREPARATIONS |
| N03AA | BARBITURATES AND DERIVATIVES |
| N03AB | HYDANTOIN DERIVATIVES |
| N03AD | SUCCINIMIDE DERIVATIVES |
| N03AE | BENZODIAZEPINE DERIVATIVES |
| N03AF | CARBOXAMIDE DERIVATIVES |
| N03AG | FATTY ACID DERIVATIVES |
| N03AX | OTHER ANTIEPILEPTICS |
| N04AA | TERTIARY AMINES |
| N04AB | ETHERS CHEMICALLY CLOSE TO ANTIHISTAMINES |
| N04AC | ETHERS OF TROPINE OR TROPINE DERIVATIVES |
| N04BA | DOPA AND DOPA DERIVATIVES |
| N04BB | ADAMANTANE DERIVATIVES |
| N04BC | DOPAMINE AGONISTS |
| N04BD | MONOAMINE OXIDASE B INHIBITORS |
| N04BX | OTHER DOPAMINERGIC AGENTS |
| N05AA | PHENOTHIAZINES WITH ALIPHATIC SIDE-CHAIN |
| N05AB | PHENOTHIAZINES WITH PIPERAZINE STRUCTURE |
| N05AC | PHENOTHIAZINES WITH PIPERIDINE STRUCTURE |
| N05AD | BUTYROPHENONE DERIVATIVES |
| N05AE | INDOLE DERIVATIVES |
| N05AF | THIOXANTHENE DERIVATIVES |
| N05AG | DIPHENYLBUTYLPIPERIDINE DERIVATIVES |
| N05AH | DIAZEPINES, OXAZEPINES, THIAZEPINES AND OXEPINES |
| N05AL | BENZAMIDES |
| N05AN | LITHIUM |
| N05AX | OTHER ANTIPSYCHOTICS |
| N05BA | BENZODIAZEPINE DERIVATIVES |
| N05BB | DIPHENYLMETHANE DERIVATIVES |
| N05BC | CARBAMATES |
| N05BE | AZASPIRODECANEDIONE DERIVATIVES |
| N05BX | OTHER ANXIOLYTICS |
| N05CB | BARBITURATES, COMBINATIONS |
| N05CD | BENZODIAZEPINE DERIVATIVES |
| N05CF | BENZODIAZEPINE RELATED DRUGS |
| N05CH | MELATONIN RECEPTOR AGONISTS |
| N05CM | OTHER HYPNOTICS AND SEDATIVES |
| N05CX | HYPNOTICS AND SEDATIVES IN COMBINATION, EXCL. BARBITURATES |
| N06AA | NON-SELECTIVE MONOAMINE REUPTAKE INHIBITORS |
| N06AB | SELECTIVE SEROTONIN REUPTAKE INHIBITORS |
| N06AF | MONOAMINE OXIDASE INHIBITORS, NON-SELECTIVE |
| N06AG | MONOAMINE OXIDASE A INHIBITORS |
| N06AX | OTHER ANTIDEPRESSANTS |
| N06BA | CENTRALLY ACTING SYMPATHOMIMETICS |
| N06BC | XANTHINE DERIVATIVES |
| N06BX | OTHER PSYCHOSTIMULANTS AND NOOTROPICS |
| N06CA | ANTIDEPRESSANTS IN COMBINATION WITH PSYCHOLEPTICS |
| N06DA | ANTICHOLINESTERASES |
| N06DX | OTHER ANTI-DEMENTIA DRUGS |
| N07AA | ANTICHOLINESTERASES |
| N07AB | CHOLINE ESTERS |
| N07AX | OTHER PARASYMPATHOMIMETICS |
| N07BA | DRUGS USED IN NICOTINE DEPENDENCE |
| N07BB | DRUGS USED IN ALCOHOL DEPENDENCE |
| N07BC | DRUGS USED IN OPIOID DEPENDENCE |
| N07CA | ANTIVERTIGO PREPARATIONS |
| N07XX | OTHER NERVOUS SYSTEM DRUGS |
| P01AB | NITROIMIDAZOLE DERIVATIVES |
| P01AX | OTHER AGENTS AGAINST AMOEBIASIS AND OTHER PROTOZOAL DISEASES |
| P01BA | AMINOQUINOLINES |
| P01BB | BIGUANIDES |
| P01BC | METHANOLQUINOLINES |
| P01BD | DIAMINOPYRIMIDINES |
| P01BE | ARTEMISININ AND DERIVATIVES |
| P01BF | ARTEMISININ AND DERIVATIVES, COMBINATIONS |
| P01BX | OTHER ANTIMALARIALS |
| P01CX | OTHER AGENTS AGAINST LEISHMANIASIS AND TRYPANOSOMIASIS |
| P02CA | BENZIMIDAZOLE DERIVATIVES |
| P02CE | IMIDAZOTHIAZOLE DERIVATIVES |
| P02CF | AVERMECTINES |
| P02DA | SALICYLIC ACID DERIVATIVES |
| P03AB | CHLORINE CONTAINING PRODUCTS |
| P03AC | PYRETHRINES, INCL. SYNTHETIC COMPOUNDS |
| P03AX | OTHER ECTOPARASITICIDES, INCL. SCABICIDES |
| R01AA | SYMPATHOMIMETICS, PLAIN |
| R01AB | SYMPATHOMIMETICS, COMBINATIONS EXCL. CORTICOSTEROIDS |
| R01AC | ANTIALLERGIC AGENTS, EXCL. CORTICOSTEROIDS |
| R01AD | CORTICOSTEROIDS |
| R01AX | OTHER NASAL PREPARATIONS |
| R01BA | SYMPATHOMIMETICS |
| R02AA | ANTISEPTICS |
| R02AB | ANTIBIOTICS |
| R02AX | OTHER THROAT PREPARATIONS |
| R03 | DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES |
| R03AB | NON-SELECTIVE BETA-ADRENORECEPTOR AGONISTS |
| R03AC | SELECTIVE BETA-2-ADRENORECEPTOR AGONISTS |
| R03AK | ADRENERGICS AND OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES |
| R03AL | ADRENERGICS IN COMBINATION WITH ANTICHOLINERGICS |
| R03BA | GLUCOCORTICOIDS |
| R03BB | ANTICHOLINERGICS |
| R03BC | ANTIALLERGIC AGENTS, EXCL. CORTICOSTEROIDS |
| R03CA | ALPHA- AND BETA-ADRENORECEPTOR AGONISTS |
| R03CC | SELECTIVE BETA-2-ADRENORECEPTOR AGONISTS |
| R03DA | XANTHINES |
| R03DC | LEUKOTRIENE RECEPTOR ANTAGONISTS |
| R03DX | OTHER SYSTEMIC DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES |
| R05 | COUGH AND COLD PREPARATIONS |
| R05CA | EXPECTORANTS |
| R05CB | MUCOLYTICS |
| R05DA | OPIUM ALKALOIDS AND DERIVATIVES |
| R05DB | OTHER COUGH SUPPRESSANTS |
| R05FA | OPIUM DERIVATIVES AND EXPECTORANTS |
| R05FB | OTHER COUGH SUPPRESSANTS AND EXPECTORANTS |
| R05X | OTHER COLD COMBINATION PREPARATIONS |
| R06AA | AMINOALKYL ETHERS |
| R06AB | SUBSTITUTED ALKYLAMINES |
| R06AD | PHENOTHIAZINE DERIVATIVES |
| R06AE | PIPERAZINE DERIVATIVES |
| R06AX | OTHER ANTIHISTAMINES FOR SYSTEMIC USE |
| R07AA | LUNG SURFACTANTS |
| R07AB | RESPIRATORY STIMULANTS |
| R07AX | OTHER RESPIRATORY SYSTEM PRODUCTS |
| S | SENSORY ORGANS |
| S01AA | ANTIBIOTICS |
| S01AB | SULFONAMIDES |
| S01AD | ANTIVIRALS |
| S01AE | FLUOROQUINOLONES |
| S01AX | OTHER ANTIINFECTIVES |
| S01BA | CORTICOSTEROIDS, PLAIN |
| S01BC | ANTIINFLAMMATORY AGENTS, NON-STEROIDS |
| S01CA | CORTICOSTEROIDS AND ANTIINFECTIVES IN COMBINATION |
| S01CB | CORTICOSTEROIDS/ANTIINFECTIVES/MYDRIATICS IN COMBINATION |
| S01CC | ANTIINFLAMMATORY AGENTS, NON-STEROIDS AND ANTIINFECTIVES IN COMBINATION |
| S01EA | SYMPATHOMIMETICS IN GLAUCOMA THERAPY |
| S01EB | PARASYMPATHOMIMETICS |
| S01EC | CARBONIC ANHYDRASE INHIBITORS |
| S01ED | BETA BLOCKING AGENTS |
| S01EE | PROSTAGLANDIN ANALOGUES |
| S01EX | OTHER ANTIGLAUCOMA PREPARATIONS |
| S01FA | ANTICHOLINERGICS |
| S01FB | SYMPATHOMIMETICS EXCL. ANTIGLAUCOMA PREPARATIONS |
| S01GA | SYMPATHOMIMETICS USED AS DECONGESTANTS |
| S01GX | OTHER ANTIALLERGICS |
| S01HA | LOCAL ANESTHETICS |
| S01JA | COLOURING AGENTS |
| S01KA | VISCOELASTIC SUBSTANCES |
| S01LA | ANTINEOVASCULARISATION AGENTS |
| S01XA | OTHER OPHTHALMOLOGICALS |
| S02AA | ANTIINFECTIVES |
| S02BA | CORTICOSTEROIDS |
| S02CA | CORTICOSTEROIDS AND ANTIINFECTIVES IN COMBINATION |
| S02DA | ANALGESICS AND ANESTHETICS |
| S02DC | INDIFFERENT PREPARATIONS |
| S03AA | ANTIINFECTIVES |
| S03BA | CORTICOSTEROIDS |
| S03CA | CORTICOSTEROIDS AND ANTIINFECTIVES IN COMBINATION |
| V01AA | ALLERGEN EXTRACTS |
| V03AA | DRUGS FOR TREATMENT OF CHRONIC ALCOHOLISM |
| V03AB | ANTIDOTES |
| V03AC | IRON CHELATING AGENTS |
| V03AE | DRUGS FOR TREATMENT OF HYPERKALEMIA AND HYPERPHOSPHATEMIA |
| V03AF | DETOXIFYING AGENTS FOR ANTINEOPLASTIC TREATMENT |
| V03AG | DRUGS FOR TREATMENT OF HYPERCALCEMIA |
| V03AH | DRUGS FOR TREATMENT OF HYPOGLYCEMIA |
| V03AK | TISSUE ADHESIVES |
| V03AN | MEDICAL GASES |
| V03AX | OTHER THERAPEUTIC PRODUCTS |
| V04CA | TESTS FOR DIABETES |
| V04CC | TESTS FOR BILE DUCT PATENCY |
| V04CD | TESTS FOR PITUITARY FUNCTION |
| V04CF | TUBERCULOSIS DIAGNOSTICS |
| V04CG | TESTS FOR GASTRIC SECRETION |
| V04CH | TESTS FOR RENAL FUNCTION |
| V04CJ | TESTS FOR THYREOIDEA FUNCTION |
| V04CL | TESTS FOR ALLERGIC DISEASES |
| V04CM | TESTS FOR FERTILITY DISTURBANCES |
| V04CX | OTHER DIAGNOSTIC AGENTS |
| V06 | GENERAL NUTRIENTS |
| V06CA | NUTRIENTS WITHOUT PHENYLALANINE |
| V06D | OTHER NUTRIENTS |
| V06DB | FAT/CARBOHYDRATES/PROTEINS/MINERALS/VITAMINS, COMBINATIONS |
| V06DC | CARBOHYDRATES |
| V06DD | AMINO ACIDS, INCL. COMBINATIONS WITH POLYPEPTIDES |
| V06DE | AMINO ACIDS/CARBOHYDRATES/MINERALS/VITAMINS, COMBINATIONS |
| V06DF | MILK SUBSTITUTES |
| V06DX | OTHER COMBINATIONS OF NUTRIENTS |
| V07AB | SOLVENTS AND DILUTING AGENTS, INCL. IRRIGATING SOLUTIONS |
| V07AX | WASHING AGENTS ETC. |
| V07AY | OTHER NON-THERAPEUTIC AUXILIARY PRODUCTS |
| V08AA | WATERSOLUBLE, NEPHROTROPIC, HIGH OSMOLAR X-RAY CONTRAST MEDIA |
| V08AB | WATERSOLUBLE, NEPHROTROPIC, LOW OSMOLAR X-RAY CONTRAST MEDIA |
| V08AC | WATERSOLUBLE, HEPATOTROPIC X-RAY CONTRAST MEDIA |
| V08AD | NON-WATERSOLUBLE X-RAY CONTRAST MEDIA |
| V08BA | BARIUM SULFATE CONTAINING X-RAY CONTRAST MEDIA |
| V08CA | PARAMAGNETIC CONTRAST MEDIA |
| V08CB | SUPERPARAMAGNETIC CONTRAST MEDIA |
| V08DA | ULTRASOUND CONTRAST MEDIA |
| V09AB | IODINE (123I) COMPOUNDS |
| V09CA | TECHNETIUM (99m Tc) COMPOUNDS |
| V09IB | INDIUM (111IN) COMPOUNDS |
| V09IX | OTHER DIAGNOSTIC RADIOPHARMACEUTICALS FOR TUMOUR DETECTION |
| V10XA | IODINE (131I) COMPOUNDS |
| V10XX | VARIOUS THERAPEUTIC RADIOPHARMACEUTICALS |
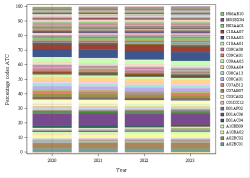
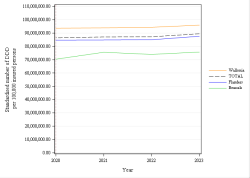
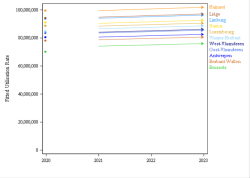
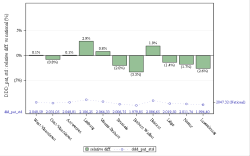
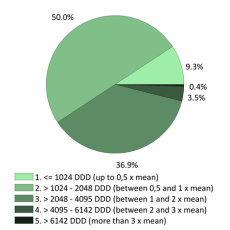
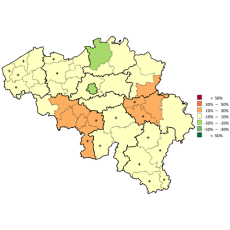
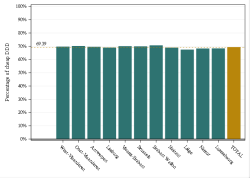
The codes mentioned above can be used in rates and expenses, or only in expenses. We invite you to consult the full report for more information.
Click below to see the graph illustrating the evolution of the breakdown by volume of ATC codes used for the rates.
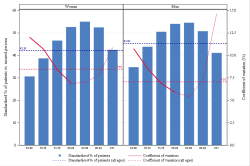
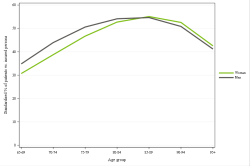
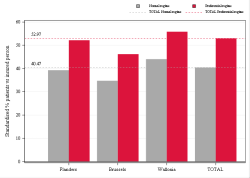
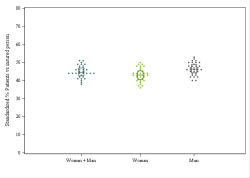
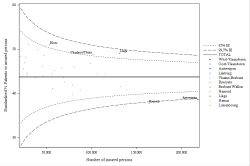
Population selection
Older than or equal to 65 years
Min 80 ddd per drug per year & min 5 drugs per patient per year
Analysis period
2023
Graphics
Comments
WE ARE INTERESTED IN YOUR OPINION
Do you have any experience in this area ? We listen to you !